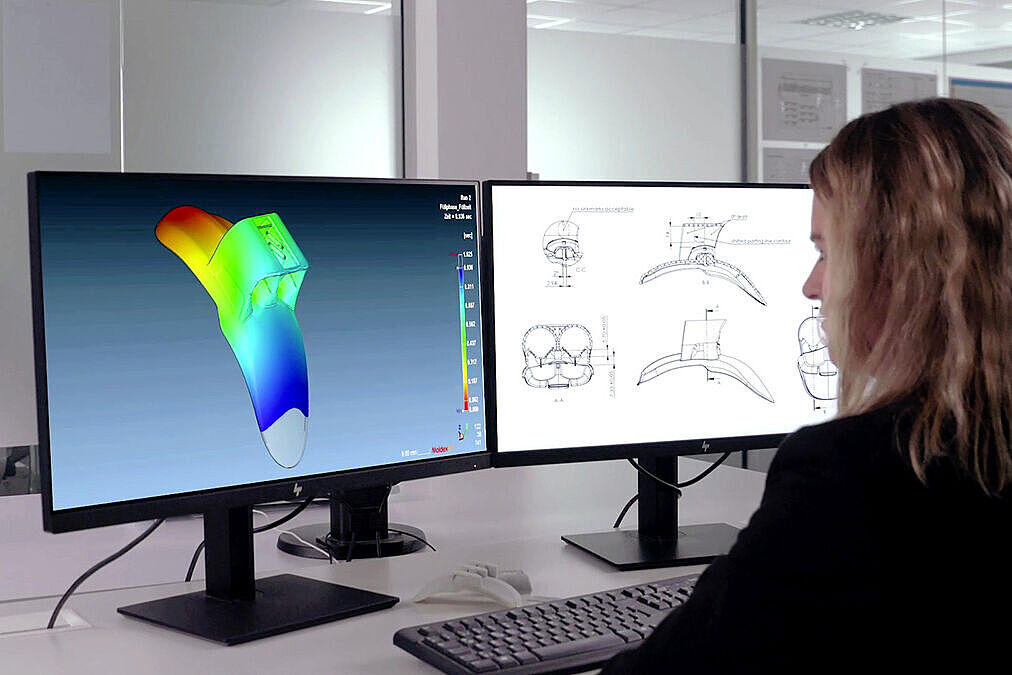
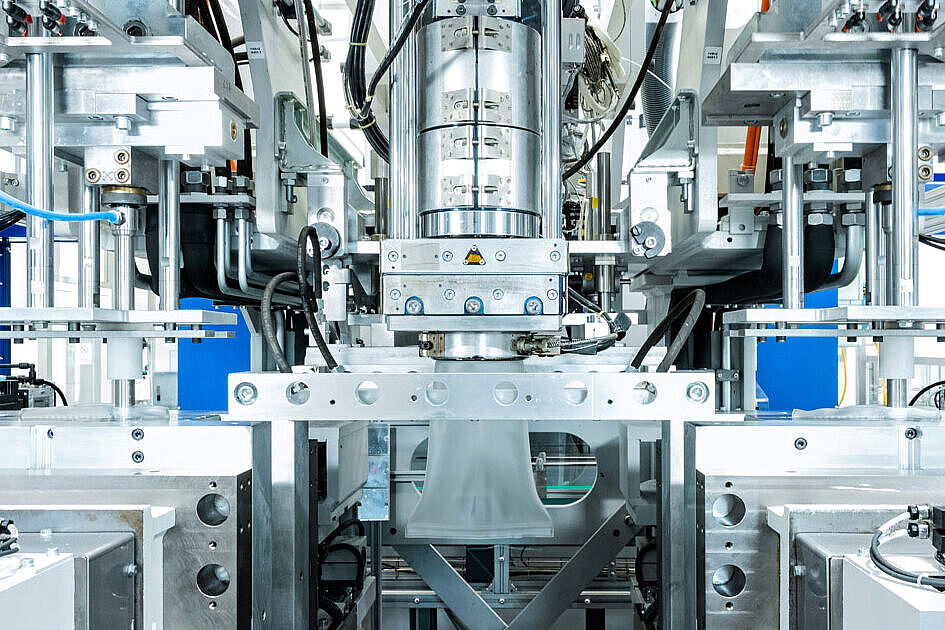
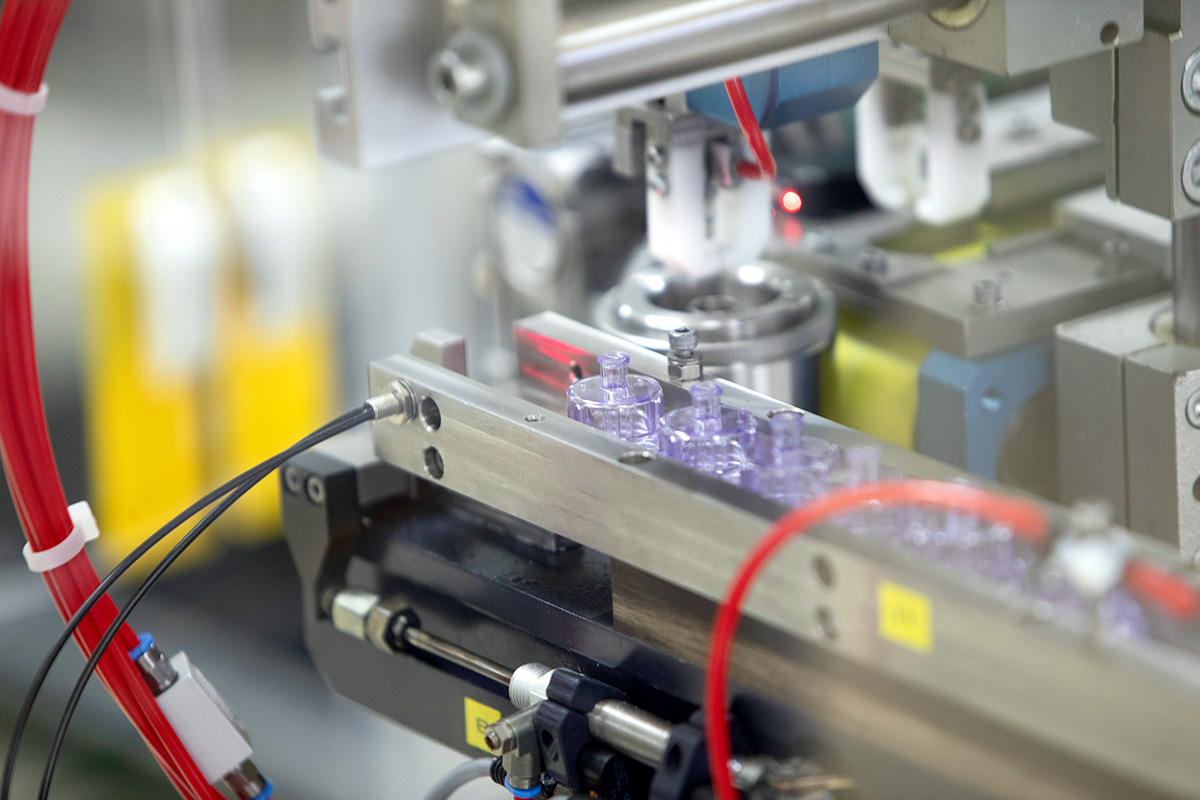
Your Partner for Development & Engineering
With our in-house, interdisciplinary team of designers, engineers, and ergonomics experts, we combine innovative strength, long-standing industry experience, and deep engineering expertise under one roof. As a CDMO (Contract Development and Manufacturing Organization), we support our customers in plastics and metal processing and are valued as a reliable development partner – whether for new product development or design optimization focused on sustainability, functionality, or cost efficiency.
From Idea to Series Production

We prefer to accompany your development process from the very beginning – from feasibility analysis, material selection, and determination of manufacturing requirements to component design, prototyping, and series production.
The earlier we are involved, the better our experts can ensure that your design is optimized from the outset in terms of functionality, quality, cost-effectiveness, sustainability, and time-to-market.
Our customers value our creative, solution-oriented approach – for example, through joint innovation workshops for idea generation – and our ability to implement concepts efficiently and transparently.
Design for Manufacturing for Plastics & Metal
The design and tolerancing of plastic components require specialized know-how to optimize processes, ensure safety, and reduce costs. Using state-of-the-art design and development tools as well as comprehensive simulations before mold construction, we ensure that every detail is perfectly aligned with function, quality, and cost efficiency. Through intelligent design and precise calculation, we create components that can be manufactured with process reliability while meeting the highest standards for stability, design, and efficiency.
Our in-depth expertise in processing plastics for medical devices, pharmaceutical packaging, and drug delivery solutions flows into every step of the process.
In addition, we have particular expertise in the United States in processing stainless steel and titanium – making us a strong partner when it comes to combining plastics and metals in medical applications. Our development teams are globally connected, creating synergies to achieve the best possible outcome for every project.
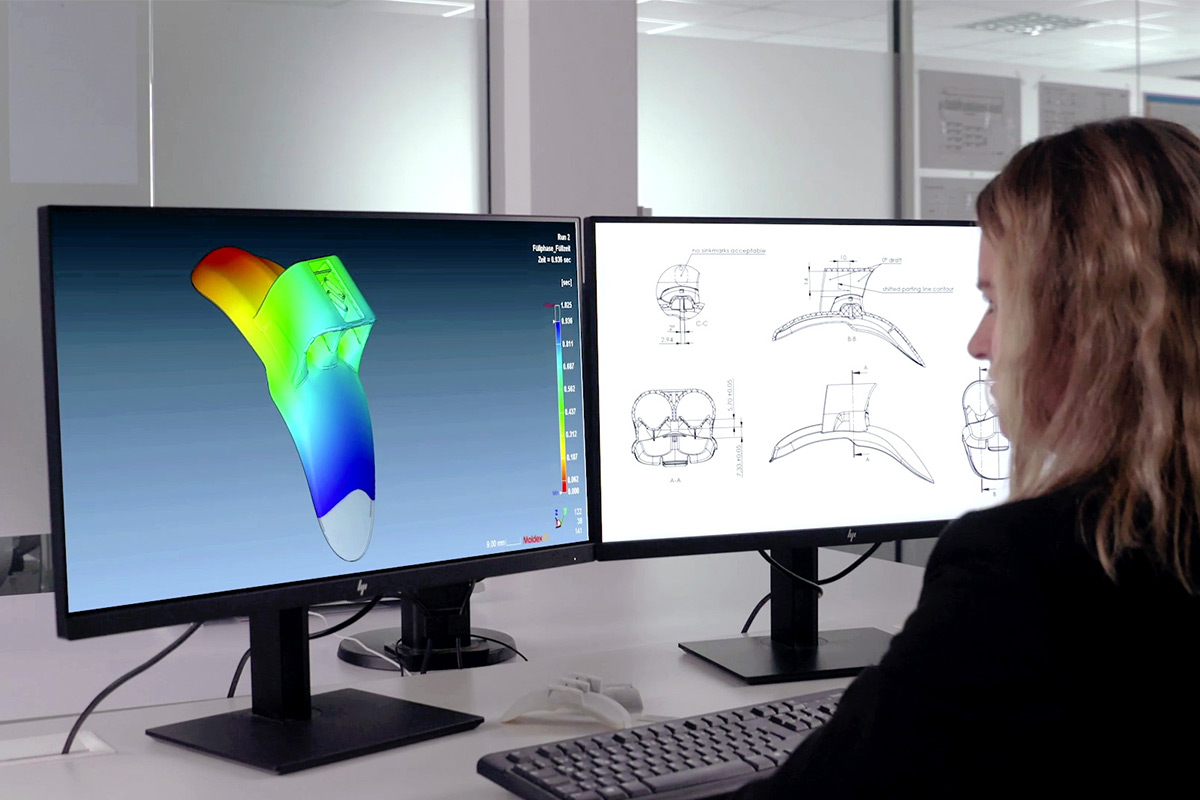
We Support Your Successful Launch
To ensure that your new development can be successfully introduced to the market, we take care of all required documentation for approval in accordance with applicable standards (ISO 13485, ISO 15378). This includes, for example, requirement specifications, design FMEAs, test protocols, and production data sheets.
Innovation at Röchling Medical
At Röchling Medical, innovation is driven by a clearly structured process that ensures new ideas are implemented purposefully, efficiently, and with foresight. This allows us to continuously expand our technological footprint and create forward-looking, sustainable solutions for our partners.
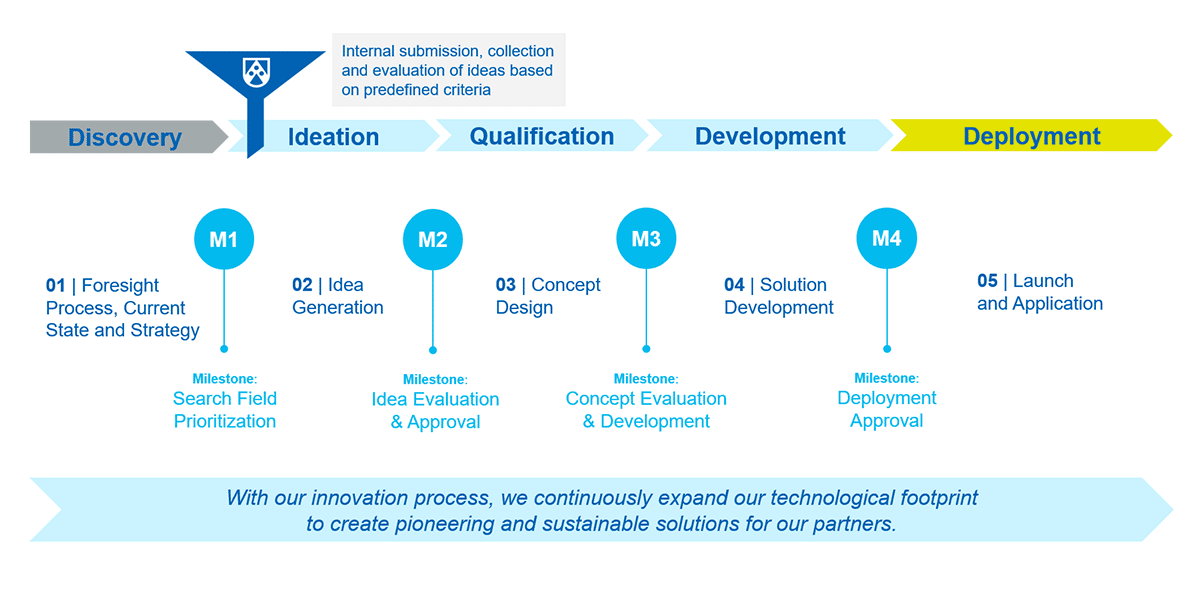
How We Live Innovation
Innovation is not a side project for us – it’s embedded in our DNA. It shapes our values and our identity – innovative, curious, and future-oriented.
That’s why we actively encourage ideas from our employees worldwide. Through our internal innovation processes, we support them from the first concept to implementation. Our ambition goes far beyond product innovation – we also drive advancements in manufacturing technologies, sustainable materials, and digital solutions. Through global collaboration and the use of cutting-edge tools, we develop solutions that make processes more efficient and have the potential to improve patient health or even save lives.
How We Innovate
Product
- Innovation workshops & talks for creative impulses and forward-thinking exchange
- Network of experts for insights
- Smart approaches to individualization
- Integration of electronics and sensors
- Digital Product Labeling (e.g. marker technology)
Material
- Advanced material combinations
- Sustainable material solutions
Process
- Smart automation solutions
- Simulations & Digital Twinning
- Accelerated prototyping solutions
- Sustainable process engineering
Customized Solutions for Individual Requirements
Our product developments are always tailored to the specific requirements of our customers. In joint formats such as InnoTalks or innovation workshops, we work together to find the optimal solution – efficient, practical, and future-proof.
Digital technologies are a key driver here: their integration into medical devices and pharmaceutical applications opens up new possibilities. The topic of digital product identification is gaining increasing importance. We possess extensive expertise in various methods and technologies that enable unique identification and secure authentication.
We also leverage our partnerships with medical experts, hospitals, universities, and research institutions to develop patient-centric solutions. This ensures that our products meet the highest standards of safety, usability, and effectiveness – today and in the future.
Sustainability is another key factor. It is becoming an increasingly central aspect of product development. With our approach based on Design-for-Sustainability (DfS), we support our customers in realizing more sustainable products – even in a highly regulated industry – without compromising on quality or safety.
Competences at the Following Locations
Case Studies
As a solution provider, we combine our expertise in research & product development, materials & technologies, and automation & industrialization with our experience, our quality standards, and our ambition to work with our customers to design a product that meets their individual requirements.
Our case studies give you an insight into the challenges faced by our customers in pharma, diagnostics and medical technology sectors and the tailor-made solutions that we jointly created for them.

Patient-Centric Pharmaceutical Packaging Design
Optimizing a Complex Class 3 Medical Device

Innovative Plastic Protectors for Glass Injection Vials

Custom Automation Concepts
Design for Manufacturability

Automated Quality Control

Metal and Plastic Processing from a Single Source
Medical Device Contract Manufacturing
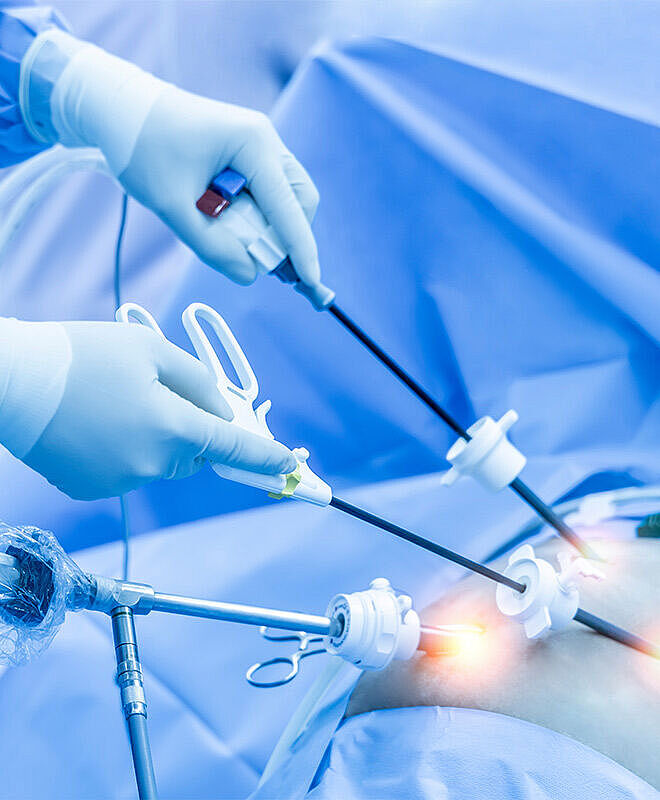
A Single Use Medical Device Designed for Sustainability
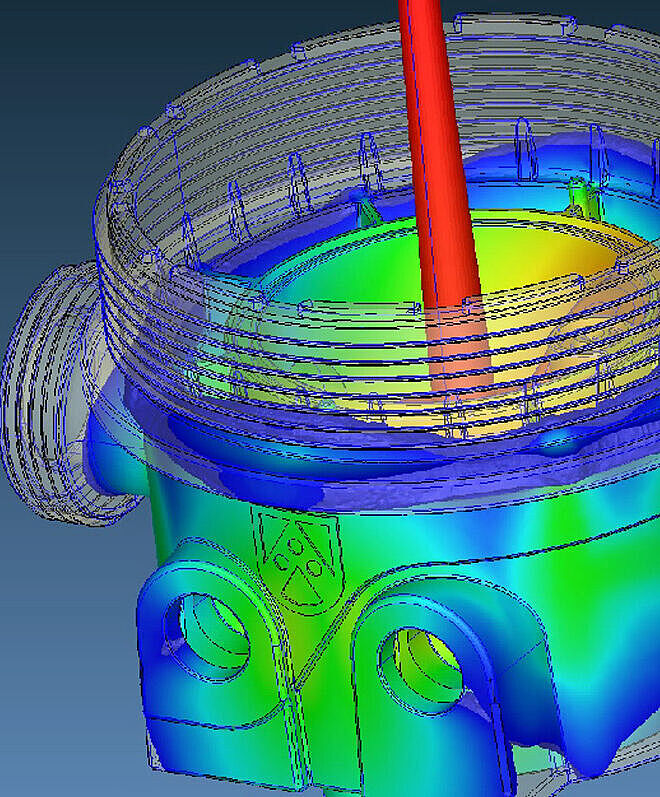
Simulations in Product Development

Medical Device for Intraoperative Radiation Therapy
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical technology and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our product design and development capabilities, please contact our team.
We look forward to hearing from you.
Your Contact in Europe

Thierry Arnaud
Vice President - Sales & Marketing Europe
Your Contact in the US

Bill Ruth
Vice President - Sales & Marketing