Development of a Single-Use Medical Device for Intraoperative Radiation Therapy
One of Germany’s leading medical technology manufacturers, headquartered in Thuringia, approached us with a clear objective: to fundamentally redesign a spherical applicator that plays a central role in an innovative approach to radiation therapy.
This advanced treatment method enables the irradiation of potentially remaining cancer cells at the margins of the tumor cavity immediately after surgical tumor removal – during the same procedure. By using a spherical applicator, the method allows for precise, localized radiation and can contribute to improved tumor control, reduced side effects, and shorter overall treatment durations compared to conventional radiation therapy.
The existing reusable applicator, made of high-performance plastic, generated high costs due to complex cleaning, resterilization, and material handling. The new single-use version was also intended to incorporate smart features such as an RFID chip to further improve traceability and product safety.
The Challenge:
- Development of a sterile single-use product to replace the reusable version
- Integration of additional features such as RFID technology
- Focus on precision, cost-efficiency, and regulatory compliance
- Comprehensive support across the entire value chain
The Path to an Efficient Single-Use Solution: Modular Design, Smart Features, and Group-Wide Synergies
Our approach was to completely rethink the product and develop a modular concept.
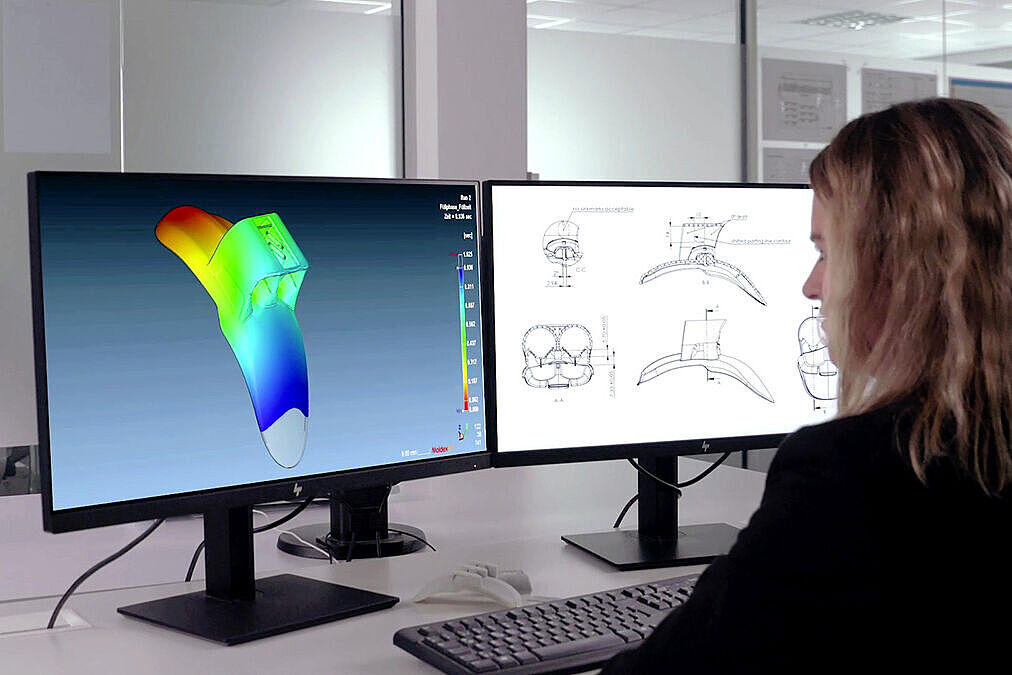
From the outset, we supported the project with extensive simulations and 3D prototypes to optimize geometries and shorten development time.
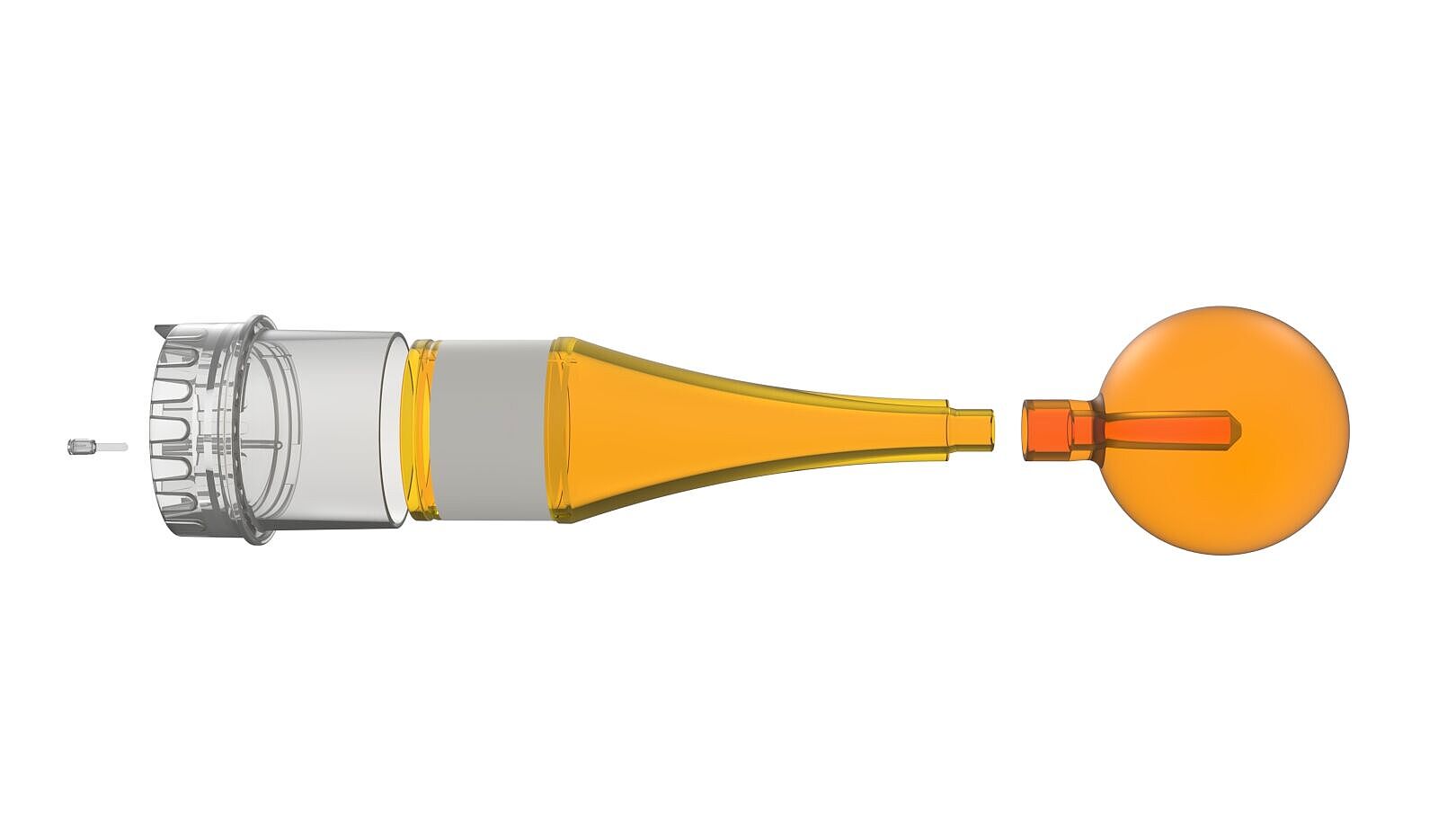
Instead of a complex, milled semi-finished part, we designed an efficient solution for the applicator’s cone — which serves as the base for all sizes — consisting of three universal injection-molded components.
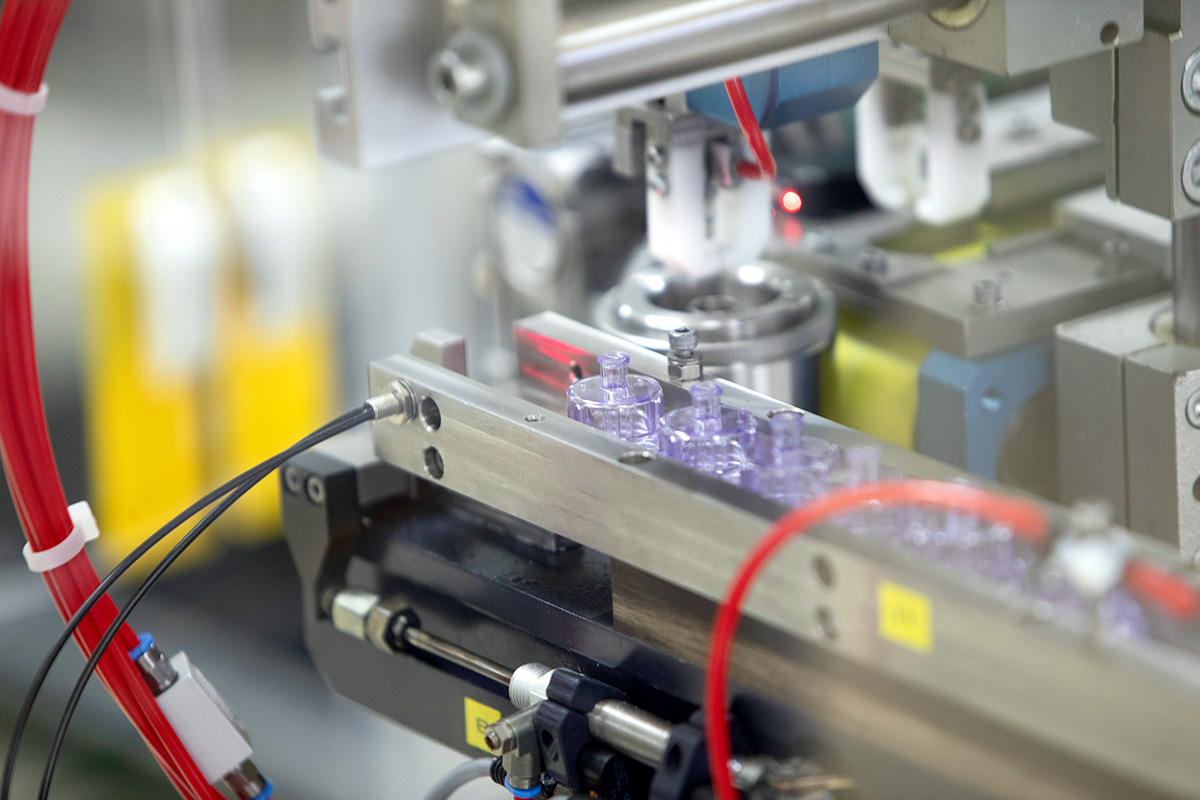
One of the product’s most critical components is the sphere. Here, we were able to leverage synergies within the Röchling Group: the material is sourced and precision-machined by Röchling Industrial. The individually sized sphere is then assembled with the cone at Röchling Medical. This approach allows us to cover the entire value chain within the Röchling Group.
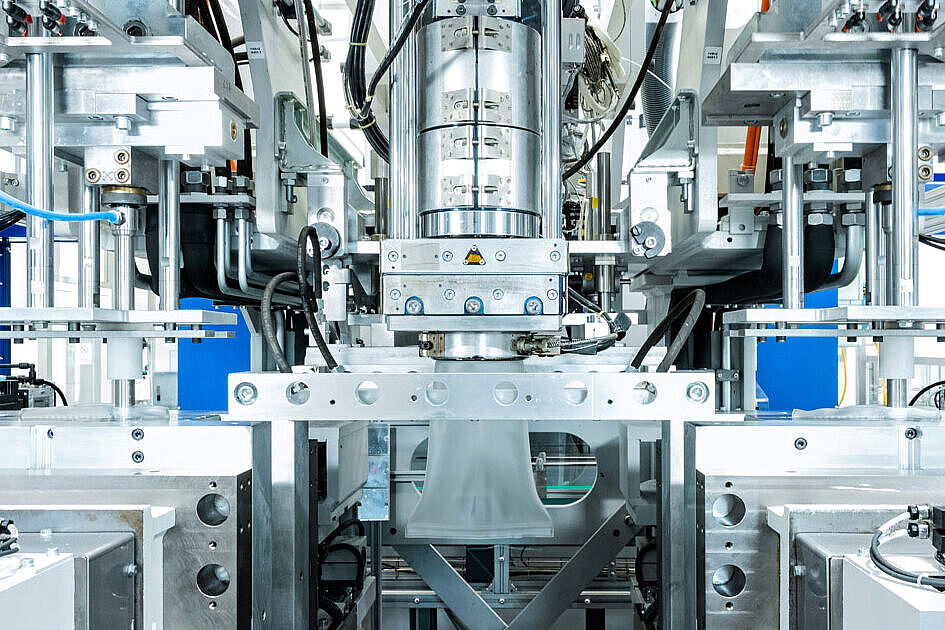
Our cleanroom production, combined with precise assembly processes — including bonding, RFID encoding, labeling, and packaging in a sterile barrier system — enables us to deliver a fully validated medical device from a single source.
Optimized OR Handling Through Precision Measuring Tools
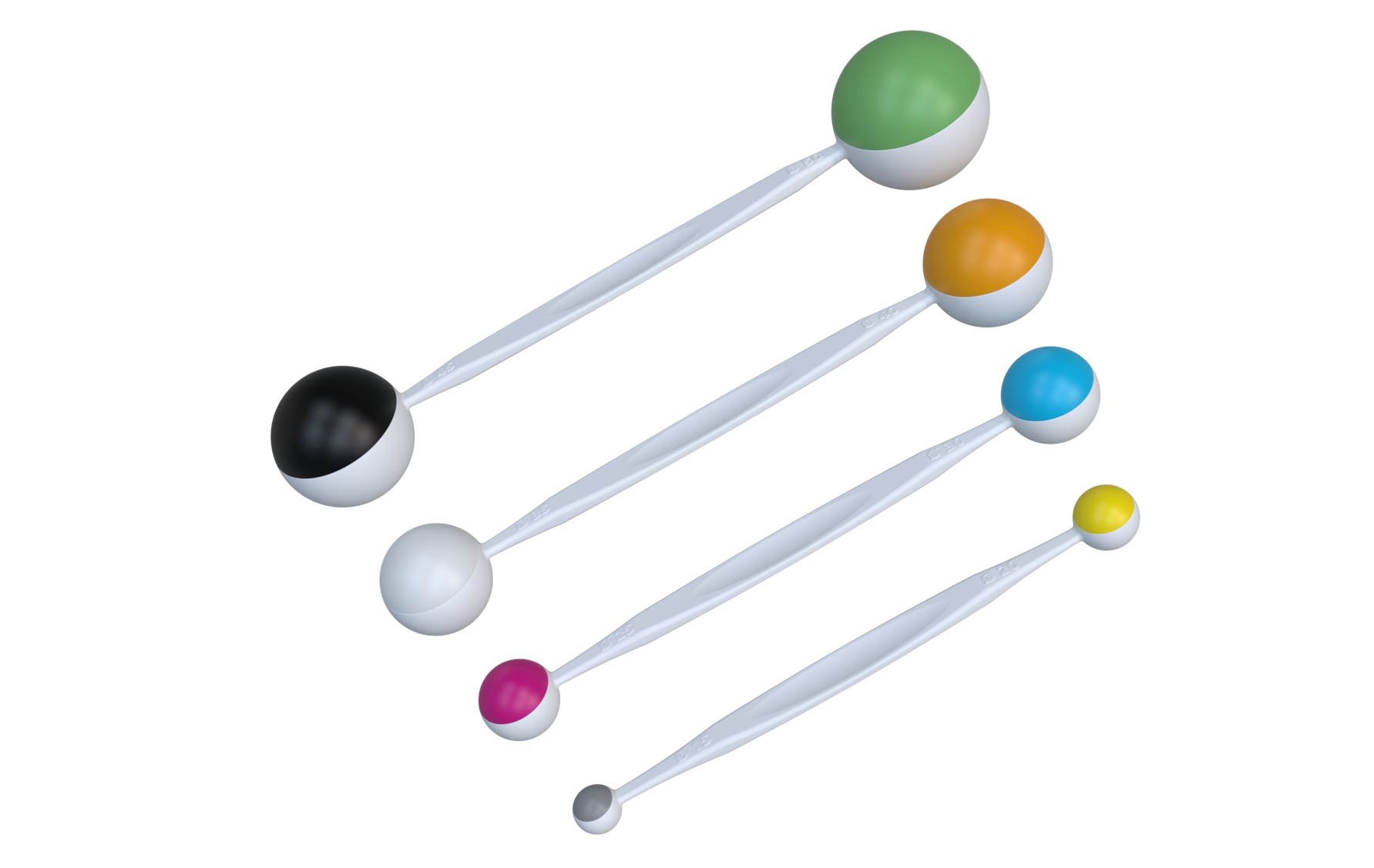
To further enhance surgical efficiency during treatment, we developed specialized measuring instruments that allow for precise determination of the tumor cavity size. This measurement serves as the basis for accurately selecting and retrieving the appropriately sized applicator from the sterile barrier system.
A clear color-coding system ensures that measuring tools, applicators, and packaging are perfectly aligned — minimizing the risk of errors during surgery and improving usability for the operating room team.
Our Approach:
- Modular concept: three universal injection-molded components and a machined sphere available in eight different sizes
- Utilization of in-house synergies for material sourcing and sphere machining
- Simulations and 3D prototyping to optimize design and reduce development time
- Development and production of specialized measuring tools for precise sizing
- Cleanroom production and assembly
- Packaging in a sterile barrier system to ensure safe application
An Innovative Single-Use Product that Combines Safety, Efficiency, and Sustainability
The result is a high-precision, sterile-packaged single-use applicator designed to significantly reduce both costs and environmental impact, while also enhancing patient safety.
A key advantage lies in our fully integrated process: thanks to our expertise in product development, cleanroom manufacturing, assembly, and finishing, we were able to deliver a complete product that meets all customer and application-specific requirements.
Special attention was given to comprehensive quality assurance and close support in regulatory affairs. In addition to providing all necessary documentation, we conducted all required validations in-house — including process, assembly, sterilization, and biocompatibility testing.
This project has allowed us to further strengthen our position as a reliable partner for complex medical devices. Our customer receives a ready-to-use product that fully complies with medical and regulatory standards — with all development and manufacturing services delivered from a single source.

The Result:
- Precise, cost-efficient single-use applicator available in eight sizes
- Full manufacturing and assembly in a cleanroom environment
- Integration of smart features such as RFID for traceability and product safety
- In-house synergies for material sourcing and machining
- Regulatory support and complete validation
- Delivery of a ready-to-use medical device from a single source
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical device and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our solutions for medical applications and customized design options, please contact our team.
We look forward to hearing from you.
Your Contact

Thierry Arnaud
Vice President - Sales & Marketing Europe